It’s great seeing that more countries are opening up to allow Singaporeans to travel to, the latest one being Thailand with its err…“unique” requirements for a tourist visa.
However, most of us know that until a successful vaccine for COVID-19 has been developed, all of our vacation plans are gonna have to be put on hold until 2050.
So for now, we’ll just have to wait for clinical trials in countries to keep tinkering with their vaccine until it’s proven to be 100% accurate. Unfortunately, when it comes to testing, there are bound to be errors which could ultimately lead to death – something that tests aim to avoid.
And recently, you might have read about how a man died from a test.
Man Died While in Clinical Trial
In Rio De Janeiro, Brazil, a 28-year-old man died while participating in the clinical trials of the COVID-19 vaccine which was developed by Oxford University and AstraZeneca, a pharmaceutical company based in Cambridge, United Kingdom.
The Washington Post reported that the man died from complications of COVID-19.
The deceased man was a physician who treated patients with COVID-19 in Rio De Janeiro.
Another unfortunate thing is this man’s death is the first to be reported among the various COVID-19 trials done worldwide.

Not much else is known about the deceased due to confidentiality requirements and clinical trial rules, said a spokesman for AstraZeneca.
This leads to the question: could there be any issue with the vaccine?
No.
Didn’t Take COVID-19 Vaccine
According to sources from Brazilian newspaper Globo and Bloomberg, the man was part of the control group which means he was given a placebo, which is just a cheem way of saying that the drug or treatment administered to him wasn’t the COVID-19 vaccine.
Clinical trials would usually involve giving both the real experimental thingy and a “fake” one (vitamins, maybe?) to two groups of people so that we can confirm that people didn’t recover due to certain issues like psychological effects, because let’s face it: some of us would magically recover from a fever after hearing that your boss is outstation.
But this is a very simplified way of explaining; to know more, you can watch this video:
Clinical Trial will Still Continue
Despite the death, the clinical trial will still continue.
The same spokesman said that events that arise from clinical trials are “assessed by trial investigators, an independent safety monitoring committee and the regulatory authorities”.
An independent committee concluded that there were no safety concerns and that testing would continue.
If there had been a severe event that could have been caused by the vaccine, then the trial would have to be paused for an investigation to take place.
Just a month ago, the same clinical trial had to be paused as one of the volunteers developed transverse myelitis which may or may not be related to the vaccination they received. Cases like this would have to be independently reviewed.
Future vaccine plans
A quick Google search will tell you that the COVID-19 situation in Brazil is, to put it bluntly, pretty bad.
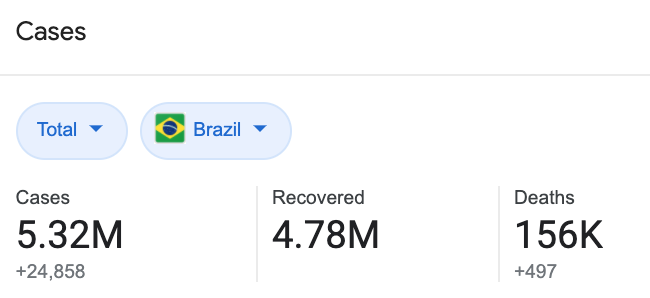
Image: Google
With over 5.3 million confirmed cases and 156,000 deaths, it’s not a wonder that clinical trials will still continue.
BBC reported that the Brazilian federal government would not buy the vaccine from China and instead reached a deal with São Paulo state to buy 46 million doses of CoronaVac.
The vaccine is currently being tested by the state’s research centre Butantan Institute before it can be used safely for the public.
In the meantime, you can read more about what would happen if we never develop a safe COVID-19 vaccine here.