Health Alert: Arsenic-Laden Eczema Cream Exceeds Safe Limits in Singapore
Are you seeking confidence and emotional relief?
The Euzema Confidence Revival Cream, targeting those suffering from eczema, claims to fulfill this need.

Finding natural remedies for eczema is a challenge, especially in Singapore, where the condition is prevalent.
Statistics from Singapore General Hospital (SGH) indicate that eczema affects approximately one in five children and one in ten adults.
This prevalence necessitates the search for suitable and affordable long-term eczema treatments.
The Malaysian-brand Euzema Confidence Revival Cream is available on its website, euzema.com, and on the e-commerce platform Carousell, priced between $50 and $100 per bottle.
It boasts being “100% all-natural herbs”, free from side effects, and steroid-free, promising a cure for eczema.
However, recent events have proven these claims to be false.

Man Suffered Purpura After Using The Eczema Cream
A man in his 30s experienced severe side effects after using the product for a year.
He developed purpura, characterized by purplish red spots resulting from small bleeds under the skin.
His doctor, suspecting arsenic-induced skin reactions, reported the case to the Health Sciences Authority (HSA).

HSA’s subsequent tests revealed that the cream contained over 430 times the permissible arsenic limits, along with high levels of betamethasone and salicylic acid.
HSA then proceeded to issue the warning on 21 Nov immediately against the use of this cream.
What’s So Toxic About It?
Arsenic, a highly toxic heavy metal, can cause skin irritations, contact dermatitis, or rashes leading to skin peeling when applied externally.
Its more harmful form, inorganic arsenic, is linked to cancers and skin lesions with prolonged dietary exposure.
Singapore’s regulation only allows a maximum of 5 parts per million (ppm) of arsenic in medicated products for topical application. However, the Euzema cream allegedly contained more than 430 times of this limit.
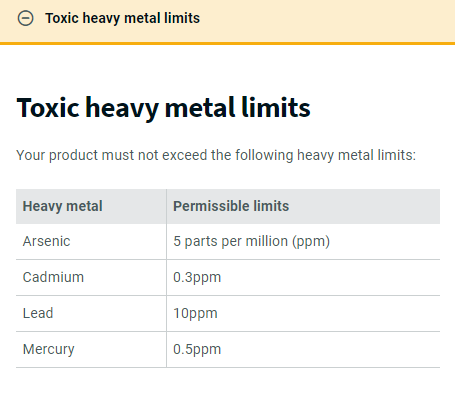
Moreover, the presence of high levels of betamethasone and salicylic acid raises additional concerns.
Betamethasone, a potent steroid, requires strict medical supervision for use. Salicylic acid, in excessive amounts, can lead to dry, irritated, and red skin, as per HSA’s guidelines.
Long-term, unsupervised steroid use may lead to high blood pressure, cataracts, increased infection risk, and Cushing’s syndrome, characterized by a round face and upper body obesity with thin limbs.
HSA also warns that abrupt cessation of steroids without medical advice can cause severe withdrawal symptoms, including adrenal insufficiency, leading to confusion, muscle and joint pain, low blood pressure, and fits or shocks.
The dangers are evident: seeking to cure eczema with products containing such heavy metals above limits might inadvertently lead to more severe health issues.
Additionally, steroids are commonly used to treat conditions like acne, psoriasis, and dandruff. Awareness of their effects and controlled usage is crucial to avoid undesirable side effects.
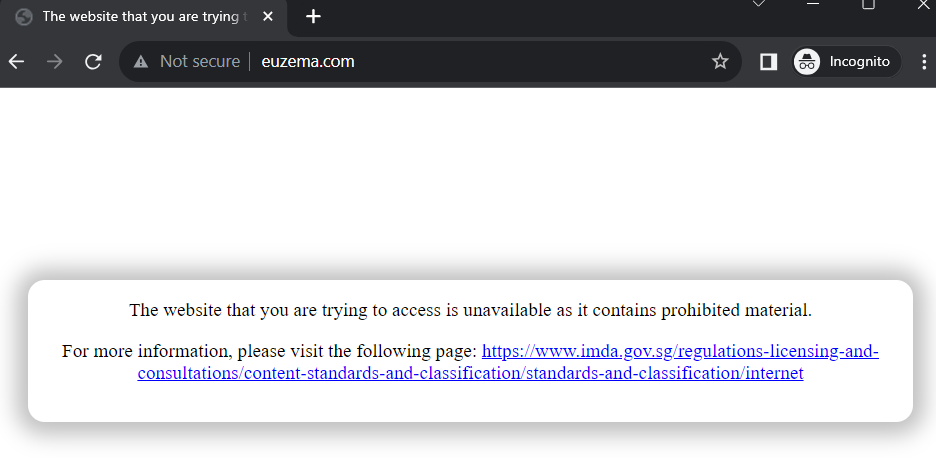
Following these revelations, Euzema’s official website has been banned for containing prohibited content. However, its Malaysian counterpart remains operational.
HSA has taken steps to remove listings from local platforms and is collaborating with Malaysian authorities, as the products are labeled as manufactured in Malaysia.
Sellers and suppliers involved face potential legal consequences, including jail time of up to two years or fines up to $10,000, or both.
FYI: Some Other Banned Products
This discovery places the cream among four products found to contain potent medicinal ingredients, alongside HONEY Q Dietary Supplement Product, SLIME 7D ADVANCE Slimming Pill, and FINOs.

These products, marketed as “herbal and natural,” were available on local e-commerce and social media platforms.

One consumer reported vomiting and headaches after using FINOs.
Other products like rash creams, coffee products, and virus-preventing medicines have also been linked to adverse effects.
Slime 7D Advance, despite claims of containing no banned substances, was found to have potent medical ingredients, including sibutramine, benzyl sibutramine, fluoxetine, and orlistat.

Sibutramine, banned in Singapore since 2010 due to heart attack and stroke risks, was previously prescribed for weight loss.
Fluoxetine and orlistat, prescription-only medicines, should be used under medical supervision.
Consumers are advised to cease using these weight loss products and seek medical attention if they experience adverse effects.
HSA emphasizes that there are no shortcuts to weight loss, advocating a balanced diet and appropriate exercise as the best approach.
It advises purchasing products only from reputable pharmacies or established retailers in Singapore.




